Chlorine Has a Higher Ionization Energy Than Aluminum
Aluminium has a density lower than those of other common metals at approximately one third that of steel. Energy increases from inner shell to the outer shells ie.
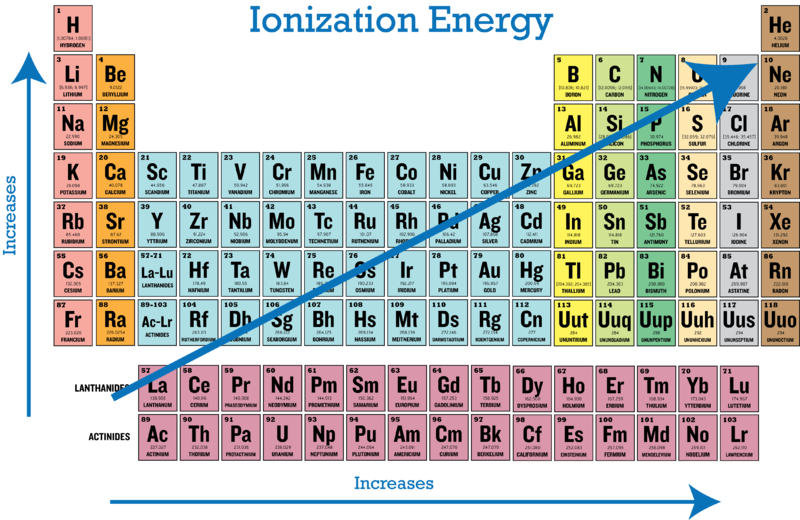
Periodic Trends In Ionization Energy Ck 12 Foundation
It has a low density good strength is easily fabricated and has excellent corrosion resistance.

. In physics and chemistry ionization energy IE American English spelling ionisation energy British English spelling is the minimum energy required to remove the most loosely bound electron of an isolated neutral gaseous atom or molecule. Select all true statements from the following. Mixture with fuels may cause explosion.
First Ionization Energy of Chlorine is. Where X is any atom or molecule X is the resultant ion when the original. Container may explode in heat of fire.
The second ionization energy of Mg is larger than the first because it always takes more energy to remove an electron from a positively charged ion than from a neutral. After 48 hr the higher concentrations were found in the stomach gastric contents ileum and duodenum followed by liver adrenal bone marrow and blood. It is ductile only when it is free of oxygen.
The first ionization energy of magnesium is larger than sodium because magnesium has one more proton in its nucleus to hold on to the electrons in the 3s orbital. Vapor explosion and poison hazard indoors outdoors or in sewers. The metal which burns in air is the only element that burns in nitrogen.
The atomic radius of an atom increases when we move down as an extra shell is. Aluminium or aluminum in American English and Canadian English is a chemical element with the symbol Al and atomic number 13. Energy for orbit nearest the nucleus is lowest.
The symbol I_1 stands for the first ionization energy energy required to take away an electron from a neutral atom and the symbol I_2 stands for the second ionization energy energy required to take away an electron from an atom with a 1 charge. Explain the variation of atomic radius along a period and down a group. During plasma etching the highly energetic and reactive species produced from a selected process gas such as O 2 or a.
Nomenclature a collection of rules for naming things is important in science and in many other situationsThis module describes an approach that is used to name simple ionic and molecular compounds such as NaCl CaCO 3 and N 2 O 4The simplest of these are binary compounds those containing only two elements but we will also consider how to name ionic compounds. Also the molecule classes covered are rather broad including for example water clusters amino acids graphene alka-. Each succeeding ionization energy is larger than the preceding energy.
Plasma etching is one of the main applications of plasma treatment and the plasma system known as a plasma etcher is commonly used in production of semiconductor devices. X X 2 e. The ionization energy associated with removal of the first electron is most commonly used.
X X e. X 2 X 3. This means energy must be supplied to a solid in order to melt it and energy is released from a liquid when it freezes because the molecules in the liquid experience weaker.
After 72 hr approximately 76 was excreted in urine and in expired air 7 as 14CO2 and less. Metals generally have lower ionization energy than nonmetals. The liquid phase has a higher internal energy than the solid phase.
Potential energy curves of rare-gas dimers isomerization energies non-covalent in-teraction energies barrier heights ionization potentials and electron a nities see also Table 1 in the supporting information. The nth ionization energy refers to the amount of energy required to remove an electron from the species with a charge of n-1. Aluminum is a chemical element with atomic number.
Hydrogen and chlorine mixtures 5-95 are exploded by almost any form of energy heat sunlight sparks etc. 1 st 2 nd and 3 rd Ionization Energies. Titanium is resistant to dilute sulfuric and hydrochloric acid most organic acids most chlorine gas and chloride solutions.
It is quantitatively expressed as Xg energy X g e. If energy is supplied then the electron moves from lower orbit to higher orbit. All nonmetal atoms release energy when forming a.
May ignite other combustible materials wood paper oil etc. Wang in Coatings for Biomedical Applications 2012 561 Plasma etching. It has a great affinity towards oxygen and forms a protective layer of oxide on the surface when exposed to.
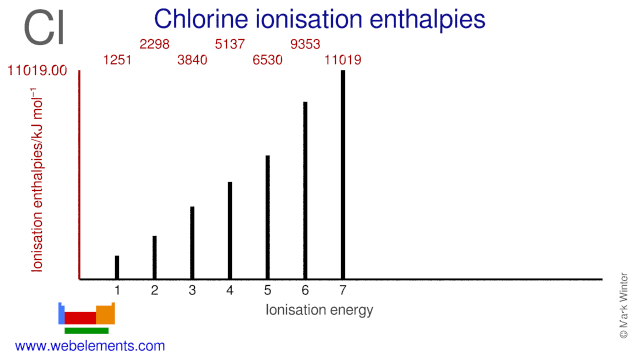
Webelements Periodic Table Chlorine Properties Of Free Atoms

Periodic Trends In Ionization Energy Ck 12 Foundation

Solved Which Of The Following Statements Is Correct Select Chegg Com
Solved Question 4 1 Points Save Answer Which Of The Chegg Com
Comments
Post a Comment